Stability of carbocation intermediates.
Vinyl carbocation definition.
The general formula for vinyl group is r ch ch 2 in which both carbon atoms are bonded with double bond and r is attached at vinylic position.
A carbocation may have one or more positive charges.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
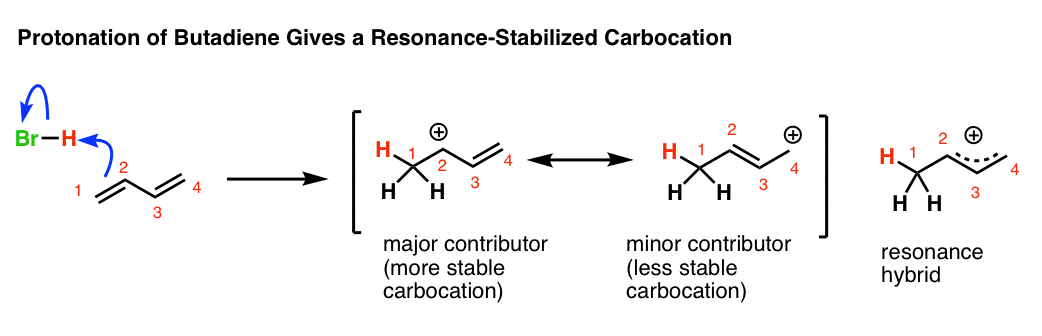
Allylic carbon atom can form stable carbocations due to electron delocalization.
Therefore carbocations are very often reactive.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
More generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized.
The carbocation carbon has sp hybridization.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
Carbocation refers to the whole molecule not only the positively charged carbon atom.
These carbocations are generally unstable because p orbitals of the carbon atom are free due to loss of electrons.
A carbocation with a two coordinate positive carbon derived from formal removal of a hydride ion h from an alkene is known as a vinyl cation.
Its empirical formula is c 2 h 3.
Because of the high s character of the orbital the positive charge resides closer to the positively charged nucleus which makes it a particularly high energy cation.
Vinylic carbocations are very unstable due to lack of p character.
In the absence of geometric constraints most substituted vinyl cations carry the formal positive charge on an sp hydridized carbon atom of linear geometry.
The term carbocation can be defined as an ion containing a positively charged carbon atom.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
Allylic carbon meaning the double bonded carbon atoms can be classified as vinylic and allylic carbon atoms.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
Since both carbon atoms form a double covalent bond so both are sp 2 hybridized.
Vinylic compounds can produce vinylic polymers such as pvc pvf pvac etc.